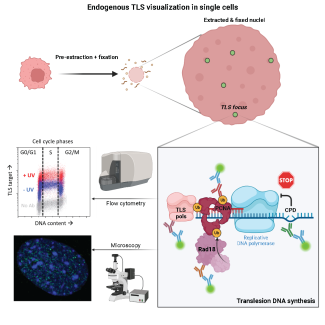
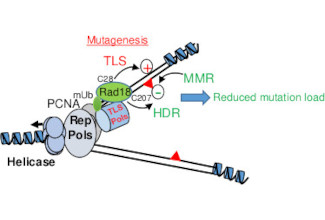
Our group is generally interested in the regulation of the DNA Damage Response (DDR), and in particular in its regulation during the early steps of embryonic development. The main function of the DDR is to slow down, or arrest cell prolferation in the presence of DNA lesions (DNA breaks, telomer integrity, replication forks stall) so to avoid cell division in the presence of DNA damage and therefore avoid the propagation of mutations that are drivers of genomic instability. A strong genomic instability is a feature of cancer cells (telomeric fusions, trabnsocations, duplications, deletions). To date it is admitted that the majority of sporadic tumours are the consequence of mutations in DDR genes, which can also lead to the development of genetic diseases. Hence the DDR is currently considered as a major barrier to malignat transformation playing a key role in maintenace of genomic stability.