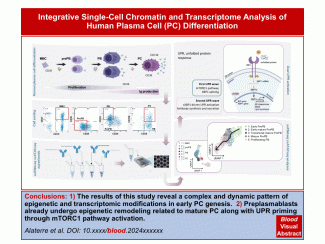
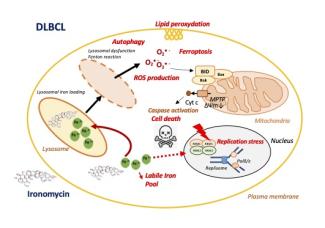
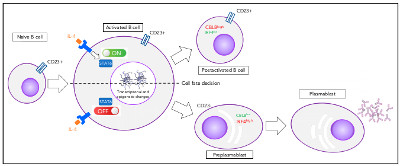
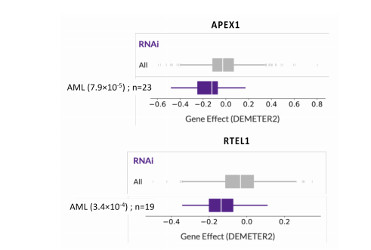
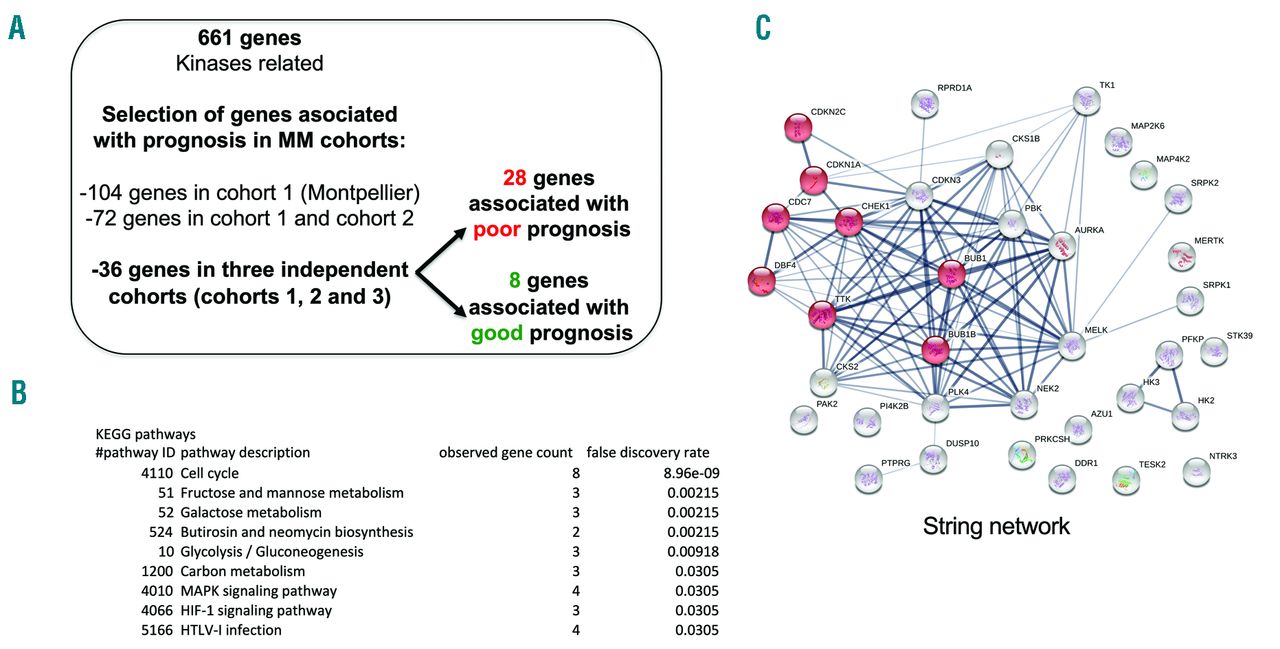
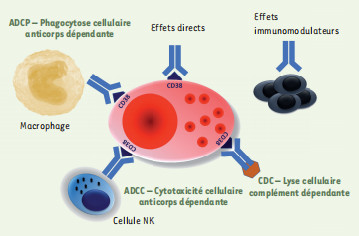
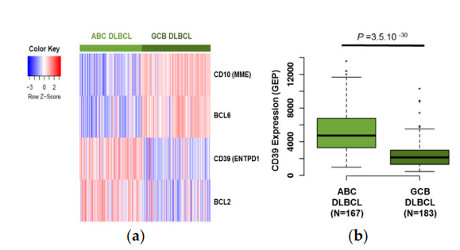
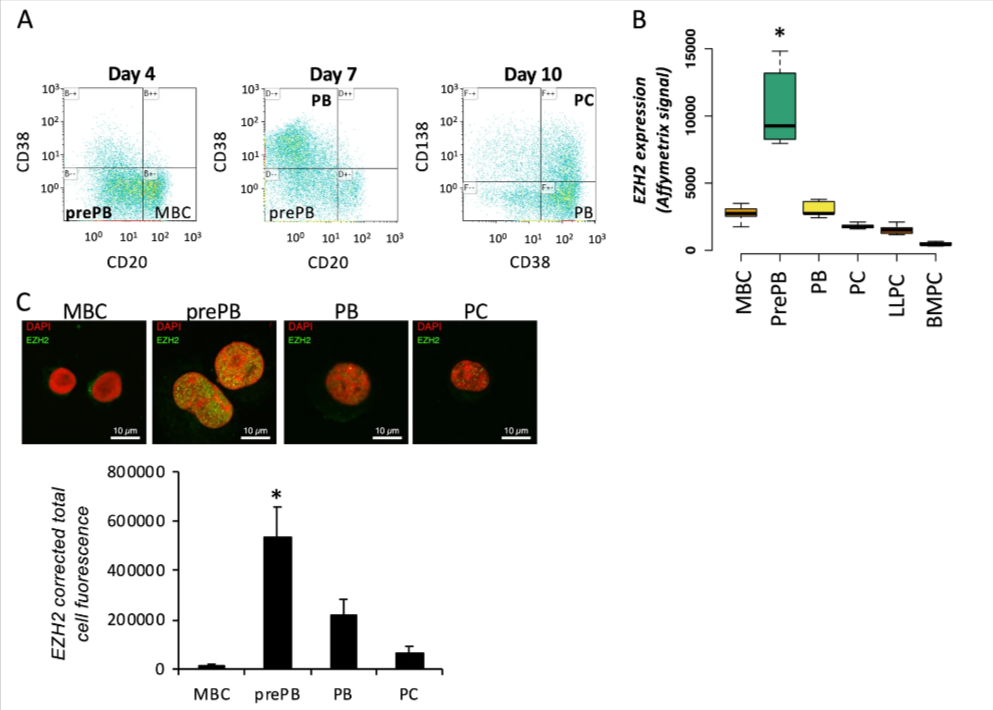
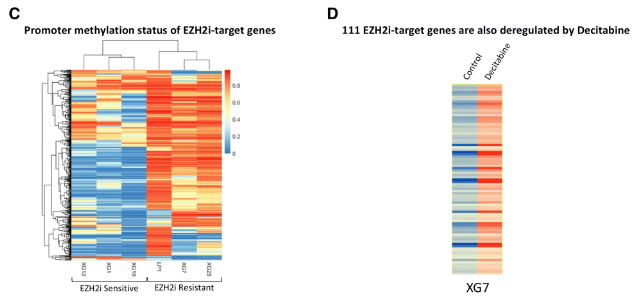
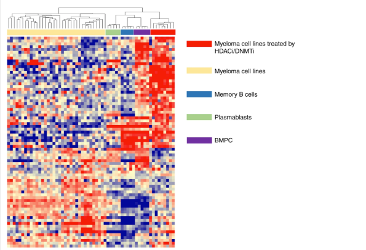
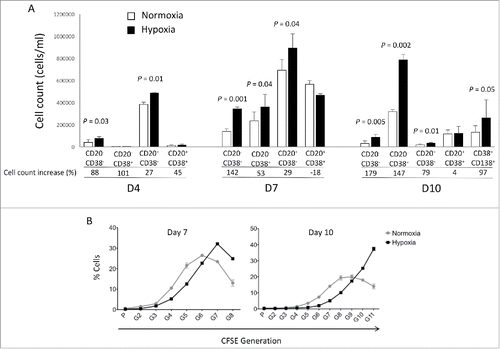
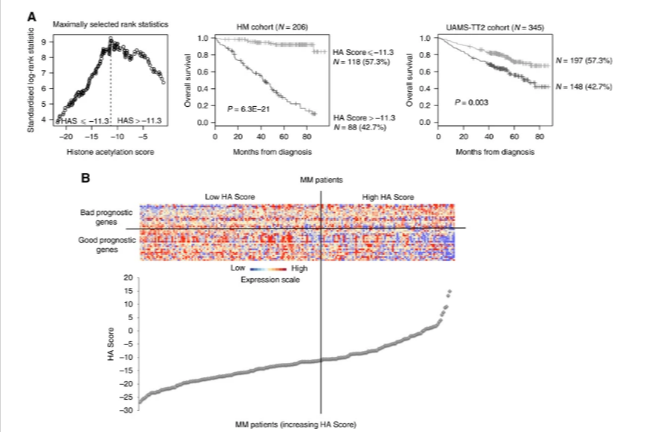
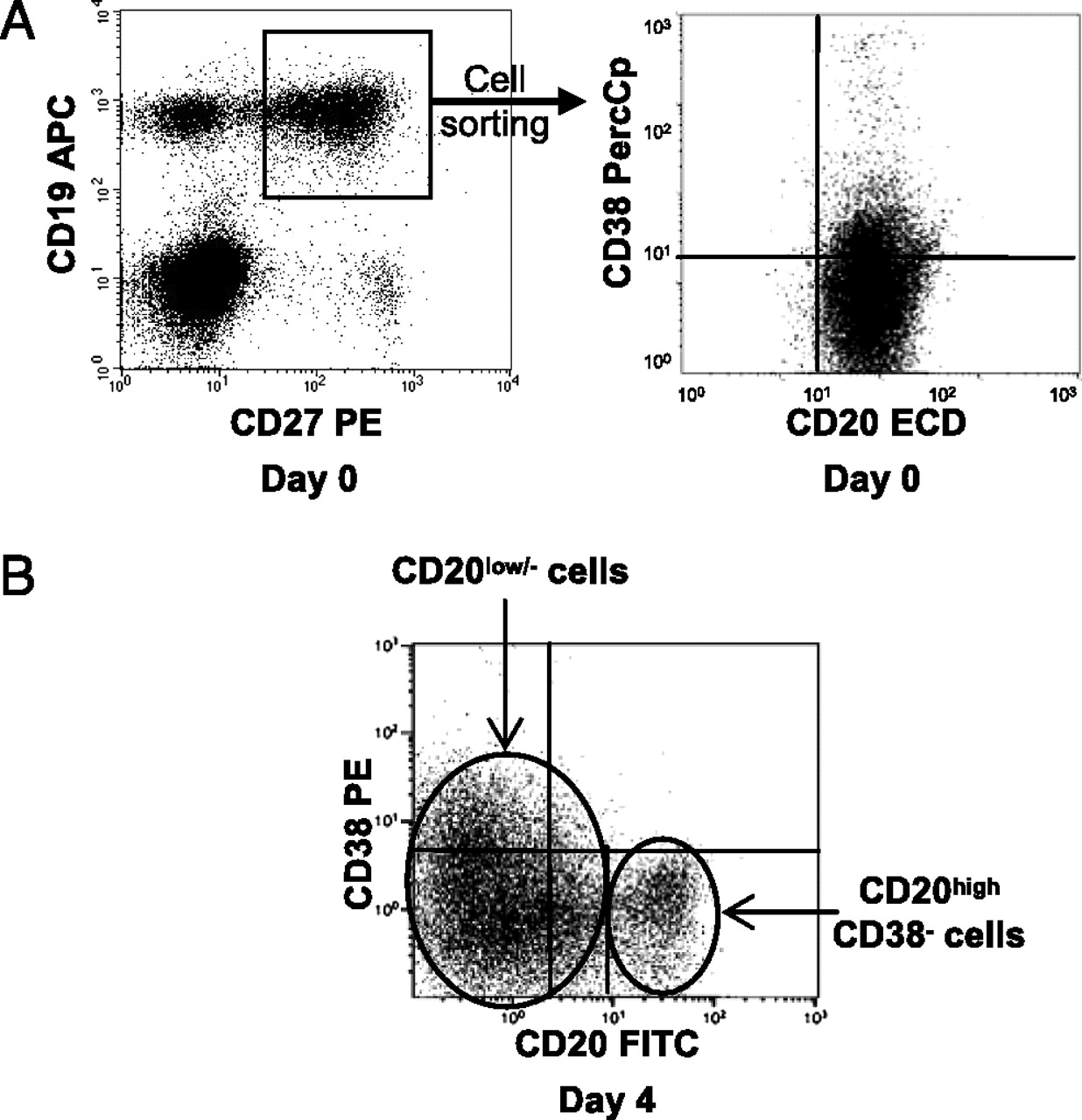
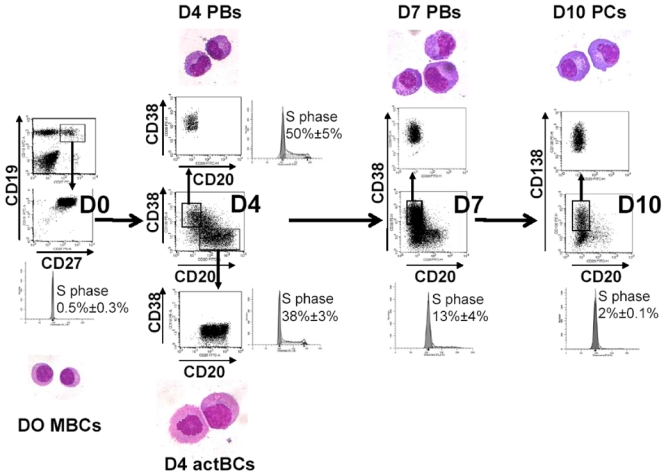
Antibody secreting cells are critical effector cells and long-lived sentinels for immune memory. B cell maturation should be tightly regulated to ensure efficient immune response without autoimmunity or immune deficiency. On the transcriptional level, the differentiation of B cells into plasma cells is associated with substantial and coordinated changes in the gene expression profile, which fall into two main categories: the loss of B cell-associated transcripts and the acquisition of plasma cell gene expression program. These changes are tightly guided by two sets of stage-specific transcription factors (TFs) that repress each other: i) B cell TFs (PAX5, BCL6 and BACH2) maintaining the B cell fate and ii) plasma cell TFs (IRF4, BLIMP1 and XBP1) that are required to extinguish the B cell genes and activate the antibody-secreting cell program. Although the role of the complex network of transcription factors involved in plasma cell differentiation has been investigated, the mechanisms regulating key plasma cell differentiation transcription networks remain poorly known. Little is known about the dynamic and hierarchical nuclear architecture in B to plasma cell differentiation and how it could regulate fundamental processes during normal plasma cell differentiation. We aim to characterize and understand the epigenetic, remodeling, structural and architectural changes that affect chromatin during B to plasma cell differentiation. Furthermore, we aim to identify the molecular events involved in tumorigenesis during B to PC differentiation.
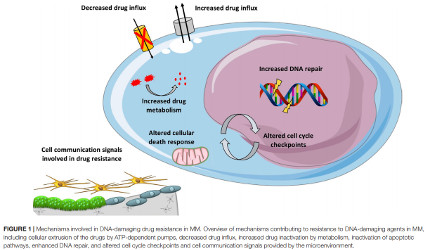
Mature B cell malignancies are genetically and clinically heterogeneous diseases. Treatment improvements will come from a better comprehension of tumorigenesis and detailed molecular analyses to develop individualized therapies that take into account their molecular heterogeneity and subclonal evolution.
Our laboratory uses genome data, computing, mathematical modeling and unique cellular models to study mature B cells and plasma cells with a focus on epigenome modifications and genomic instability. These approaches work in tandem with technological advancements to study tumorigenesis and to understand the mechanisms of tumor progression and drug resistance to develop new diagnostic and treatment strategies.
These projects are developed in collaboration with other groups of the IGH together with the departments of biological and clinical hematology of the university hospital of Montpellier. Taking advantage of the link with the laboratory of monitoring innovative therapies (Montpellier University Hospital), dedicated to diagnosis and residual disease monitoring of patients with plasma cell neoplasms, we aim to strongly foster a transformation of basic research into translational and clinical applications.
We created a startup company Diag2Tec to valorize the research activity of the group.