Project : The role of distal regulatory elements in alternative splicing regulation
01/06/2016
Alternative splicing (AS) is a highly regulated process that is essential for creating the protein diversity required for correct cell-type specification. Dysregulation of AS programs can lead to cellular dysfunction, developmental problems and disease, such as cancer. Understanding the manner in which alternative splicing is regulated during development and disease is therefore of the utmost importance.
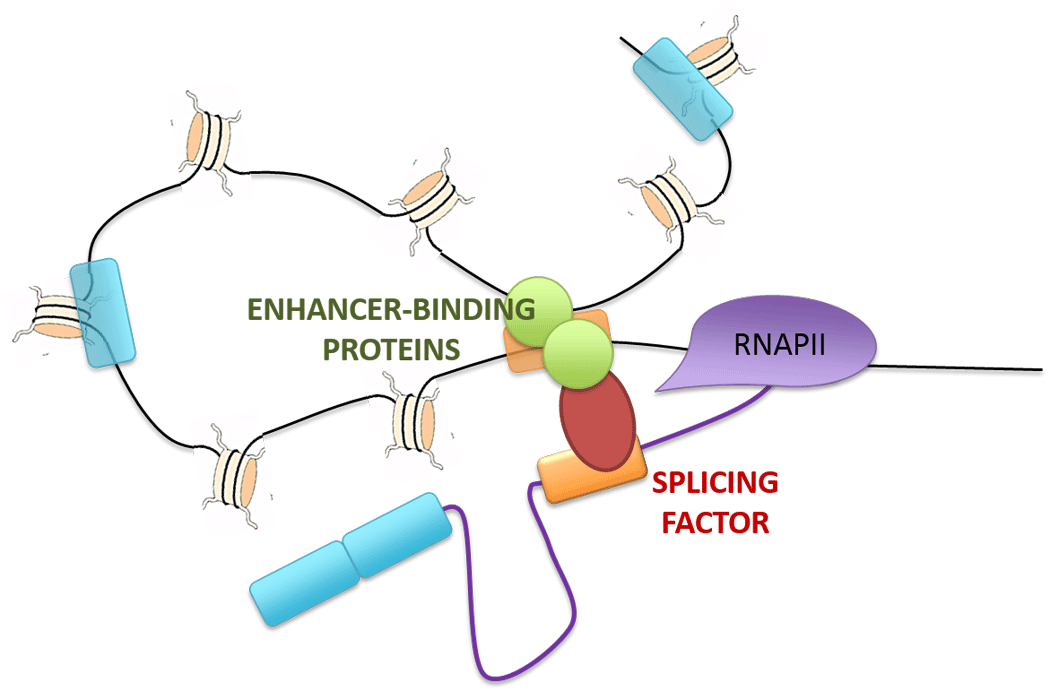
Recent advances in the field have shown that, not only does AS regulation rely on the recruitment of various splicing factors to the pre-messenger RNA, but other phenomena such as RNA Polymerase II elongation rate, histone post-translational modifications or long non-coding RNAs have been shown to regulate this process. Based on recent findings that alternatively spliced exons can physically interact with distant genomic regions through tridimensional chromatin looping, we hypothesize that distal regulatory elements (DREs) also play a critical role in the establishment and maintenance of cell-type-specific alternative splicing programs. To this end, as an ongoing project initiated in the Luco lab (https://institut-curie.org/team/luco), we are studying the effect of DRE activity on AS regulation during the epithelial-to-mesenchymal transition (EMT), an important physiological process that is involved in tumor metastasis and cancer recurrence. Using genome-wide studies, Marie-Sarah Cabrillac (PhD student)* and Andrew Oldfield (team leader) have mapped and physically linked active DREs to intragenic regions of alternatively spliced genes over the course of the EMT in human mammary epithelial cells. We are now employing a candidate-based approach to tease apart the multiple processes through which DRE activation or decommission can regulate cell-type-specific splicing patterns in order to discover new DRE-linked regulators implicated in the alternative splicing process.
As a long-term goal, this project aims to establish a direct functional link between DRE activity and AS during development, with the ultimate ambition of being able to modify alternative splicing patterns through the regulation of DREs. This study will yield results expected to have important implications for the understanding of how cancer-specific splicing programs are established and maintained and have the potential to discover innovative targets to revert EMT and in this way reduce tumor metastasis and cancer progression.
*Marie-Sarah Cabrillac: PhD student from the Luco lab, co-supervised by Andrew Oldfield and Bernd Schuttengruber.