Project : Regulation of genome stability during early embryogenesis
It has been known for a longtime that the DDR is inefficient in the early embryos, however the reasons for this regulation and the underlying molecular bases are poorly understood. We have explored this issue in the early embryos of fruit fly Drosophila melanogaster, the clawed frog Xenopus laevis as well as in mouse embryonic stem cells (ES cells).
In Xenopus, we have discovered that the DDR is inefficient because the embryos efficiently by-pass DNA lesions by constitutive activation of the DNA damage tolerance pathway involving translesion DNA synthesis (TLS). This regulation limits replication fork stalling in front of DNA lesions and therefore DDR activation (Figure 1). We have explored the consequences of constitutive TLS activation on genome stability during embryonic development. We have observed a very high mutation rate as well as the presence of large DNA rearrangements that depend on TLS. We have also determined the consequences of such mutations throughout development in Drosophila. We have found that absence of the TLS DNA polymerase eta (Pol eta) during the earliest stages of embryonic development results in reduced viability at the larva stage, and in decreased genetic variability on pericentromeric heterochromatin in the adults. We also found that the mutagenic signature of TLS Pol eta identified in Drosophila is similar to that found in certain human cancers (Figure 2, Lo Furno et al., Nucleic Acids Research, in press). This constitutes a novel mechanism of genetic variation operating during very early development contributing to the individual genetic polymorphism and may explain the predisposition to develop cancer. These findings also pave the way to understand the molecular basis of genome instability observed in human embryos.
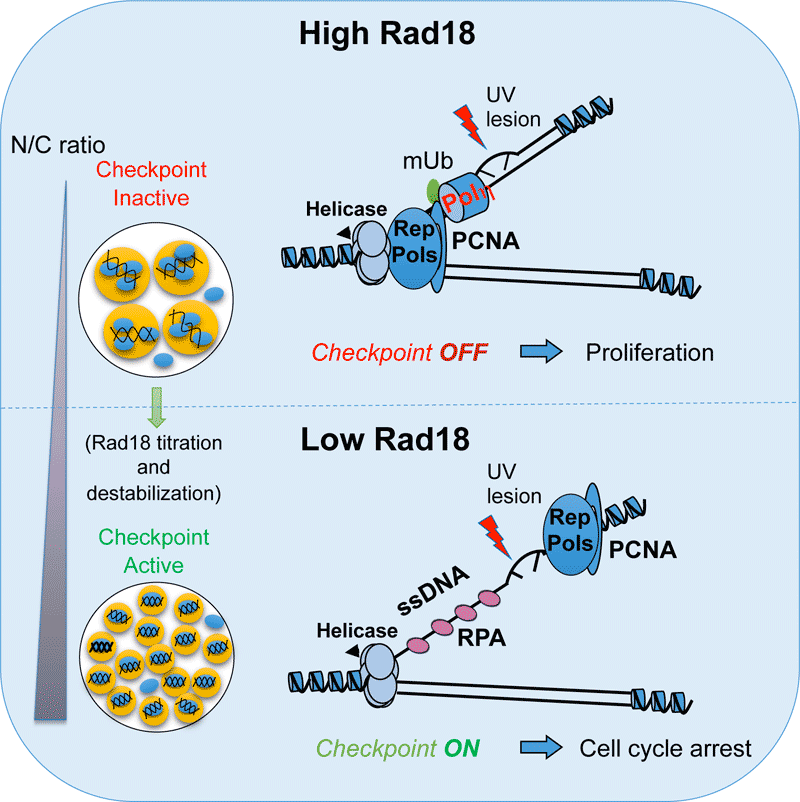
Figure 1. Constitutive activation of translesion DNA synthesis inhibts DDR activation during early Xenopus development. (Adapted from Kermi et al., Dev Cell 2015).
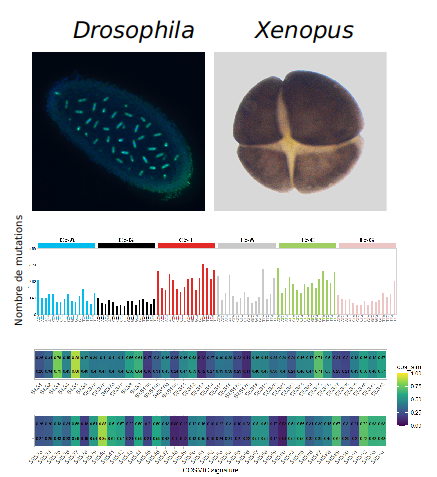
Figure 2. Upper panels, very early Drosophila and Xenopus embryos. Middle panel, embryonic mutational signature of TLS Pol eta. Lower panel, Heatmap showing the normalized relative contribution of TLS Pol eta mutational signatures from human tumors identified in COSMIC database.
Mouse embryonic stem cells (ES) also display an inefficient DDR for the G1/S checkpoint and by consequence show several signs of genomic instability. We have discovered that in these cells the G1/S checkpoint is inefficient because the critical G1/S regulator, the CDC25A protein phosphatase, is very abundant. We have also identifed the molecular bases of this abundance by showing that its stability depends upon the Dub3 ubiquitine hydrolase whose expression is under control of two pluripotency factors, Esrrb and Sox2 (van der Laan et al., 2013 Mol Cell and Figure 2). We are currently investigating the molecular basis of genomic instability of ES and iPS cells to improve their use in regenerative medicine.
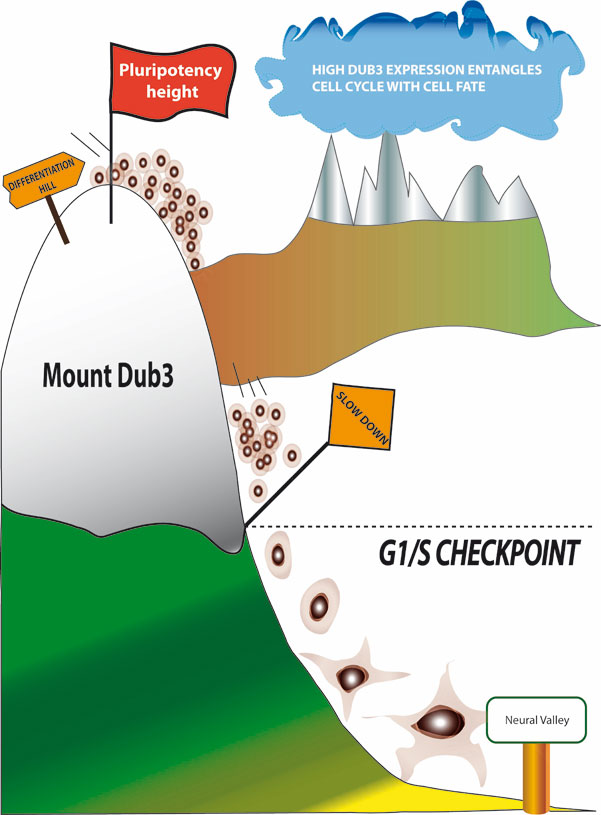
Figure 3. Cartoon showing how Dub3 expression controls activation of the G1/S checkpoint and the pluripotent state of mouse ES cells.

