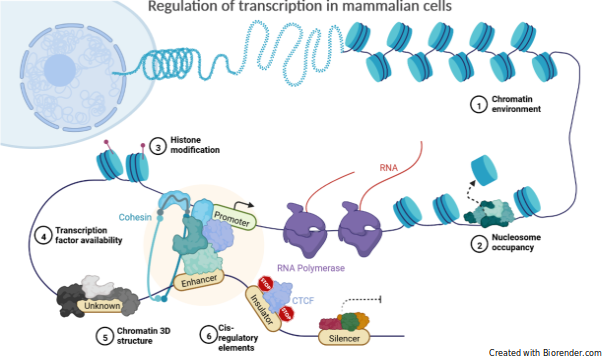
Project : The study of mesenchymal-specific enhancer activation during EMT initiation
Cell-type-specific activation of enhancers is due to a specific subset of transcription factors (TFs) termed pioneer factors, which can associate with nucleosomal or heterochromatic DNA, preceding and commonly also enabling occupancy of other TFs via nucleosome repositioning, chromatin modifier recruitment, and/or enhancer protection from DNA methylation. Each cell-type has its combination of pioneer factors/master regulators which enables the correct patterning of enhancer activity necessary to implement major developmental fate decisions and specific cell identities. Through the indispensable task of generating accessible chromatin domains during development, these proteins are directly responsible for some of the earliest events involved in transcriptional reprogramming.
In humans, despite the existence of several hundreds of thousands putative enhancers, how only a small subset is specifically activated in a given cell-type is still unclear. Mapping active enhancers, identifying by whom, how and when they are activated, and which genes they regulate, are essential to understand how cell determination is established or hijacked, in detrimental cases like cancer.
With this project, our goal is to elucidate how mesenchymal-specific distal regulatory elements (DREs) are activated during the epithelial-to-mesenchymal transition (EMT) and study their impact on this cell identity change.
Concretely, our aims are the following:
-
Aim 1: Identify the genes targeted by mesenchymal-specific (M-spe) DREs and establish how their 3D DRE-promoter interactions evolve during the EMT (in collaboration with Tom Sexton, IGBMC, Strasburg).
-
Aim 2: Determine which proteins are responsible for M-spe DRE activation, the kinetics of their actions, and understand how their activity is specific to this subset of binding sites.
-
Aim 3: Validate the candidate proteins’ roles in the EMT process.
-
Aim 4: Perform a mechanistic dissection of a subset of M-spe DREs to confirm our genome-wide findings and identify the precise DNA regions responsible for these enhancers’ accessibility and activity.
-
Aim 5: Demonstrate an in vivo role for our mesenchymal-specific DRE landscape and its effect on the mesenchymal cell transcriptome in patient-derived primary breast cancer tissues (in collaboration with E. Charafe-Jauffret & C. Ginestier, CRCM, Marseille).
By uncovering the actors, the mechanisms and the target genes of cell-type-specific DREs during the EMT, we hypothesize that the gained knowledge will enable us to disrupt the epithelial-to-mesenchymal transition. This will be done by rewiring the EMT enhancer landscape, thus preventing the initiation of the mesenchymal gene expression program and in this manner, block detrimental EMT phenotypical changes. Long-term it has the ambition of providing novel therapeutic targets to inhibit the EMT and reduce tumour metastasis and chemoresistance.