Projet : ERC Advanced Grant 2017 3DEpi
01/11/2018 - 31/10/2023
Transgenerational epigenetic inheritance of chromatin states: the role of Polycomb and 3D chromosome architecture
Start date: 2018-11-01, End date: 2023-10-31
Institution: CNRS, Institute of Human Genetics, Montpellier, France (IGH)
Project duration: 60 Months
Abstract: Epigenetic inheritance entails transmission of phenotypic traits not encoded in the DNA sequence and, in the most extreme case, Transgenerational Epigenetic Inheritance (TEI) involves transmission of memory through multiple generations. By transiently enhancing long-range chromatin interactions, we established isogenic Drosophila epilines that carry stable alternative epialleles, defined by differential levels of the Polycomb-dependent H3K27me3 mark. These are ideal systems to study the role of Polycomb group (PcG) proteins and other components in regulating nuclear organization and epigenetic inheritance of chromatin states. The present project conjugates genetics, epigenomics, imaging and molecular biology to reach three critical aims.
Aim 1: Analysis of the molecular mechanisms regulating Polycomb-mediated TEI. We will identify the DNA, protein and RNA components that trigger and maintain transgenerational chromatin inheritance as well as their mechanisms of action.
Aim 2: Role of 3D genome organization in the regulation of TEI. We will analyze the developmental dynamics of TEI-inducing long-range chromatin interactions, identify chromatin components mediating in 3D chromatin contacts and characterize their function in the TEI process.
Aim 3: Identification of a broader role of TEI during development. TEI might reflect a normal role of PcG components in the transmission of parental chromatin onto the next embryonic generation. We will explore this possibility by establishing other TEI paradigms and by relating TEI to the normal PcG function in these systems and in normal development.
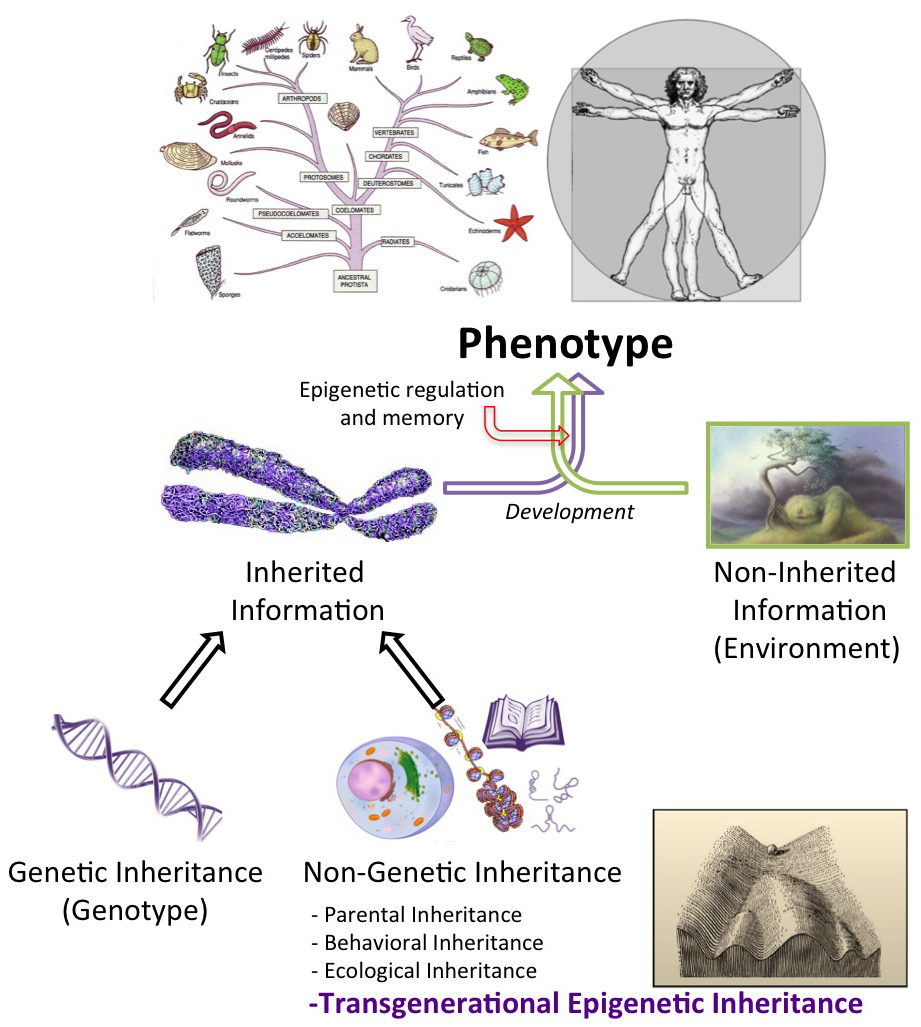 The information stored in our genome is tightly intertwined with its function. In some cases, cells may pass on to their progeny their functional state. This transmission of cellular memory has been dubbed epigenetic inheritance [1]. This phenomenon is very important during development and physiology. In the most spectacular way, it extends to inheritance of a phenotypic trait into subsequent generations, a phenomenon for which Conrad H. Waddington provided initial evidence some sixty years ago [2]. Transgenerational Epigenetic Inheritance (TEI) can thus be defined as the transmission of phenotypic traits not encoded in the DNA sequence through multiple generations. In this context, “epialleles” might be defined as the unit carriers of heritable epigenetic information. Understanding TEI is one of the most important challenges in epigenetic research [3] (Figure 1).
The information stored in our genome is tightly intertwined with its function. In some cases, cells may pass on to their progeny their functional state. This transmission of cellular memory has been dubbed epigenetic inheritance [1]. This phenomenon is very important during development and physiology. In the most spectacular way, it extends to inheritance of a phenotypic trait into subsequent generations, a phenomenon for which Conrad H. Waddington provided initial evidence some sixty years ago [2]. Transgenerational Epigenetic Inheritance (TEI) can thus be defined as the transmission of phenotypic traits not encoded in the DNA sequence through multiple generations. In this context, “epialleles” might be defined as the unit carriers of heritable epigenetic information. Understanding TEI is one of the most important challenges in epigenetic research [3] (Figure 1).
Figure 1. The factors contributing to the phenotypic traits of an organism. Heritable information, an important determinant of the phenotype, can be genetic (DNA sequence), or non-genetic. In the cases described as of today, Transgenerational Epigenetic inheritance (TEI) may depend on non-coding RNAs, DNA modification or chromatin components. However, some TEI reports have been questioned and, in general, a mechanistic understanding of TEI is lacking.
Although evidence for TEI has been published before, the presence of confounding factors might explain some of the results [4]. TEI phenomena were mostly observed in plants and linked to DNA methylation [5-7]. When observed in animal species, they were often unstable [8-12], mediated by RNAs or DNA methylation [13-15], or the role of DNA sequence was not excluded [16]. Strong evidence for true TEI has been provided very rarely in animals [17], leaving the question of the relevance of chromatin-mediated TEI in animals open.
Among putative candidate chromatin components that might drive TEI are Polycomb and Trithorax group (PcG, TrxG, respectively) proteins. The first Polycomb (Pc) mutation was identified 70 years ago and the gene was first characterized 40 years ago. Since then, many other genes collaborating or counteracting Pc were discovered and a large body of research was devoted to understanding their biological significance [18]. PcG and TrxG proteins are evolutionarily conserved chromatin components that mediate epigenetic memory of gene regulation during development [19, 20]. In Drosophila, they bind to regulatory elements called Polycomb response elements (PREs) (or CpG islands in mammals) [21]. Once bound, they modify their surrounding chromatin and interfere with chromatin remodeling in order to regulate gene expression [19-21]. In addition to regulating the genes close to their binding sites, we showed that these proteins can establish regulatory interactions among chromatin regions located at large distances in the linear genome that coalesce in the three-dimensional space of the cell nucleus [21].
These proteins have been previously implicated in TEI in Drosophila and in C. elegans [10, 12, 22, 23]. However, inheritance was limited and diluted after few generations, preventing detailed mechanistic investigation. Recently, a role for the polycomb mark H3K27me3 has been identified in imprinting of a limited number of mouse genes [24], suggesting that Polycomb-mediated TEI is evolutionarily conserved. We previously suggested the possibility that perturbation of nuclear architecture might induce transgenerationally heritable chromatin changes [12]. We recently explored this model system and demonstrated that PRE-containing transgenes can induce stable and reversible TEI [25]. This system is powerful because it is stable and reversible, and it allowed us to exclude the role of confounding factors such as DNA sequence variation. Below, I describe how building on our results will bring answers to long-standing questions in the field. Completion of specific aims described hereafter should profoundly impact our understanding of TEI.
Background, foundation of the project and preliminary data
Chromatin and its higher-order organization play an essential role in genome regulation. DNA and histones can be extensively modified in a way that impinges on gene expression and may be inherited as well as erased upon specific regulatory cues. Furthermore, chromatin fibers can be folded into higher-order structures and chromosomes are confined in discrete “territories” [26-30]. Polycomb components are involved at each level of chromatin folding, from post-translational histone modification all the way up to regulation of global chromosome architecture (Figure 2). In our previous work using Drosophila lines carrying a transgene with a PRE from the Fab-7 region of the Hox cluster called bithorax complex (BX-C), we showed that the transgene establishes 3D contacts with the endogenous BX-C locus and that perturbation of 3D chromatin architecture affected Polycomb function in a heritable way [12].
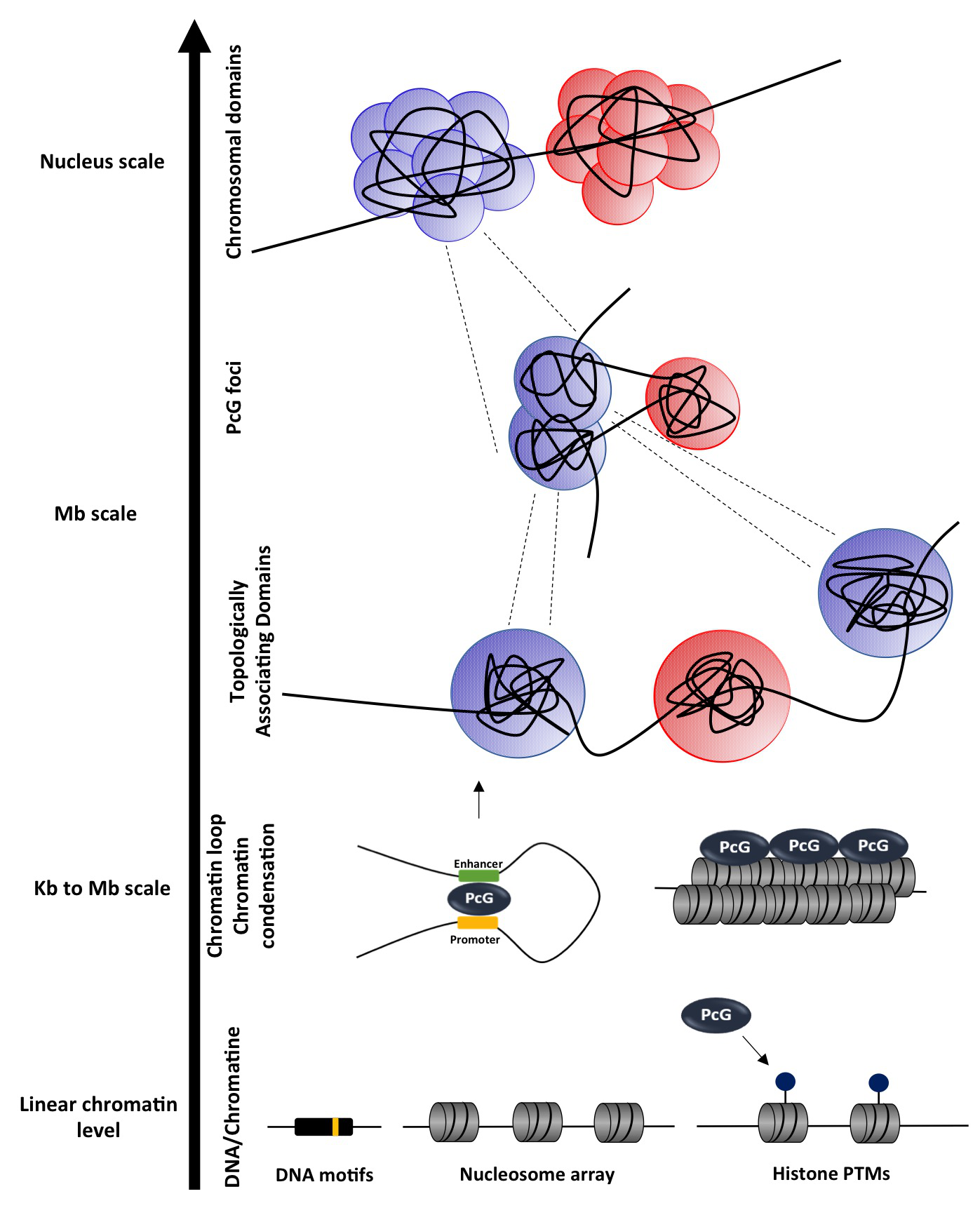 Figure 2. Role of Polycomb in genome regulation. Polycomb complexes are responsible for the deposition of the PRC2-dependent H3K27me3 and the PRC1-dependent H2AK119Ub marks, both involved in gene silencing. Furthermore, they frequently bind to large regions that are called Polycomb domains and constitute one of the fundamental and most abundant types of chromatin in the genome. Individual binding sites within domains cluster together, guiding the maintenance of each domain [31, 32]. Polycomb domains coincide with one type of Topologically associating domains that have been identified by genome-wide technologies such as Hi-C [29, 33-35]. Finally, Polycomb proteins guide and assist long-range contacts between Polycomb domains that can be very far in the linear chromosome or even in different chromosomes [36-38].
Figure 2. Role of Polycomb in genome regulation. Polycomb complexes are responsible for the deposition of the PRC2-dependent H3K27me3 and the PRC1-dependent H2AK119Ub marks, both involved in gene silencing. Furthermore, they frequently bind to large regions that are called Polycomb domains and constitute one of the fundamental and most abundant types of chromatin in the genome. Individual binding sites within domains cluster together, guiding the maintenance of each domain [31, 32]. Polycomb domains coincide with one type of Topologically associating domains that have been identified by genome-wide technologies such as Hi-C [29, 33-35]. Finally, Polycomb proteins guide and assist long-range contacts between Polycomb domains that can be very far in the linear chromosome or even in different chromosomes [36-38].
In the Fab2L line, the transgene contains a PRE flanked by a reporter gene called mini-white, which is responsible for eye pigmentation. The PRE transgene partially represses the reporter gene, such that Fab2L flies have low levels of eye pigmentation that are variable between individuals, a phenomenon that reflects metastable silencing in somatic cells. These somatic epigenetic differences are not transgenerationally heritable, as self-crossing flies with the most repressed or the most derepressed eye phenotypes does not cause any phenotypic shift in the progenies [25]. However, a transient perturbation of nuclear organization can induce TEI in this line. We removed one copy of the endogenous Fab-7 element by crossing Fab2L flies with a derivative carrying the transgene in the absence of the endogenous Fab-7 copy. This induces an increase in the contact frequency between the transgenic locus and the single remaining copy of the endogenous Fab-7 in the F1. We then restored the missing endogenous Fab-7 and obtained an F2, which is genotypically and phenotypically the same as the P0 parental generation. F2 flies with the most repressed and the most derepressed eye color were then segregated in two distinct groups, whose progenies were subjected to selection based on the eye color. Strikingly, starting at the F3, we observed an increase of the number of pigmented flies in the “derepressed” group and an increase of the number of flies with reduced pigment levels in the “repressed” group. This trend was amplified over generations, finally reaching the establishment of two “epilines” (Fab2L White* for the repressed case and Fab2L Red* for the derepressed case), which stably maintain their phenotypic trait for at least 50 generations (Figure 3). The system is reproducible and the same results was obtained on another transgenic line called FabX [12] and upon isogenizing the Fab2L line in a different genetic background [25].
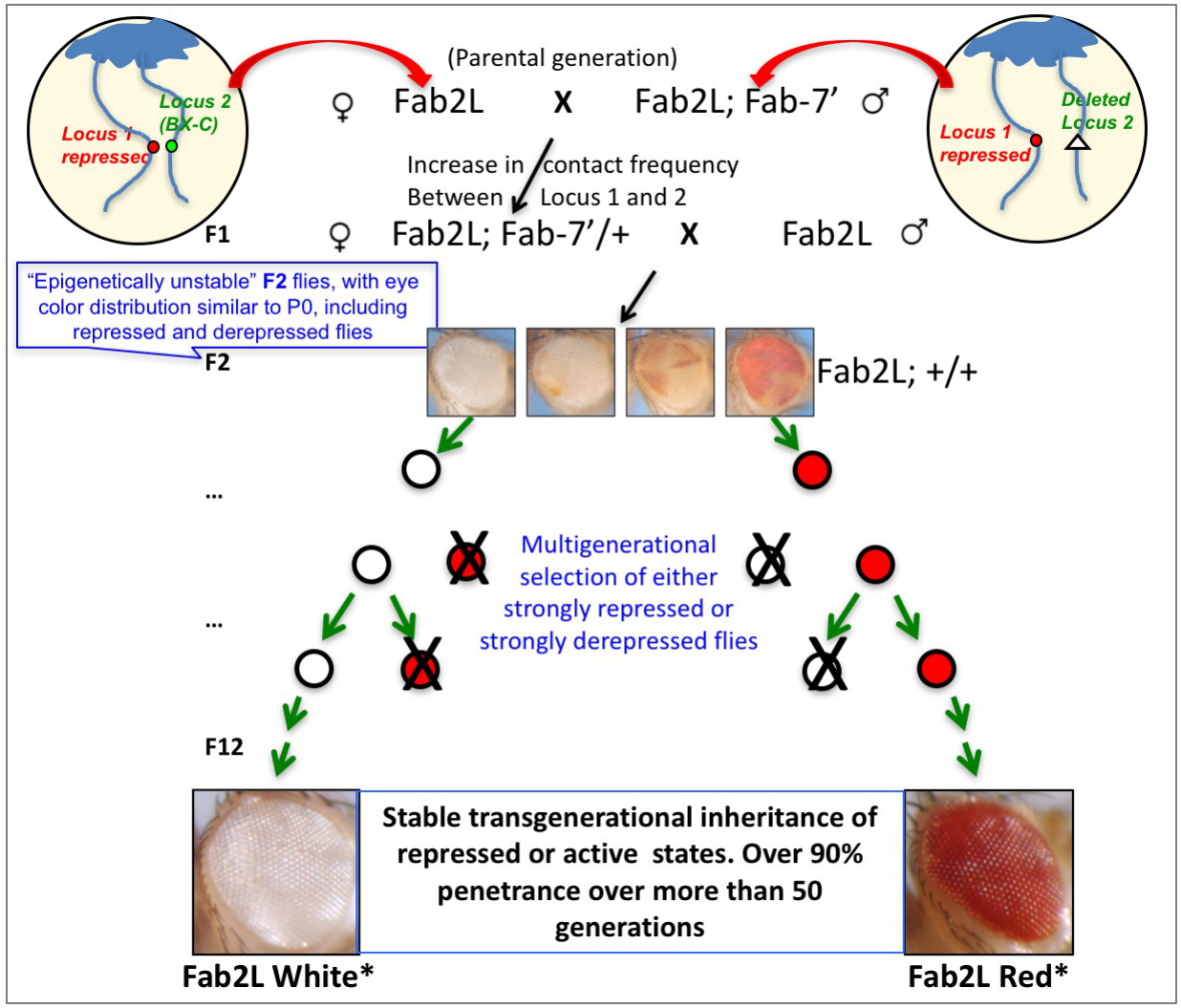 Figure 3. Establishment of stable Drosophila epilines that can transmit Polycomb-dependent chromatin states through multiple generations
Figure 3. Establishment of stable Drosophila epilines that can transmit Polycomb-dependent chromatin states through multiple generations
Furthermore, the epigenetic nature of TEI was confirmed by the findings that the epilines could be reversed by genetic manipulation and that genomic sequencing of the different epilines did not reveal differences in chromatin genes or in genes linked to eye pigmentation. Instead, we found a significant difference in the levels of H3K27me3 at the PRE-containing transgene among the epilines and a transient reduction in the dosage of the enzymatic component of PRC2, E(z), induced a heritable derepression of the transgene. Environmental changes, notably in the temperature at which the flies are exposed, can modulate the expressivity of the epialleles. Finally, we extended our paradigm to a naturally occurring phenotype that depends on Hox gene deregulation [25]. These data provide a clear-cut demonstration for TEI in animals [39] and, most importantly they provide a formidable model system for the molecular dissection of TEI, thanks to the establishment of epilines with stable and strong phenotypic differences compared to their naïve counterparts.
Project aims
What are the molecular mechanisms regulating TEI? How is this phenomenon linked to chromosome architecture and nuclear organization? How is it linked to mitotic inheritance of chromatin architecture? Finally, how widespread is TEI in biology and in response to environmental challenges? In this project, we will exploit the power of Drosophila as well as CRISPR manipulation of mouse ES cells in order to address these three questions. Specifically, the proposal has three aims:
- Aim 1: Analysis of the molecular components regulating Polycomb-mediated TEI. We will identify the DNA, protein and RNA components leading to transgenerational chromatin inheritance and we will analyze their mechanisms of action. In particular, we will analyze how can PcG binding set up a heritable chromatin structure. In addition to experiments assessing transgenerational inheritance, we will also ask how cells can mitotically inherit chromatin architecture and what is the contribution of Polycomb components in this process. For this purpose, we will use mouse ES and differentiated cells.
- Aim 2: Role of nuclear organization in the regulation of TEI. We will analyze the developmental dynamics of TEI-inducing long-range chromatin interactions, identify chromatin components mediating in 3D chromatin contacts and characterize their function in the TEI process. Furthermore, we will address mitotic inheritance of 3D chromatin organization in mouse ES and differentiated cells.
- Aim 3: Identification of a broader role of TEI during development. TEI might reflect a normal role of PcG components in the transmission of parental chromatin onto the next embryonic generation. We will explore this possibility by establishing other TEI paradigms and by relating TEI to the normal PcG function in these systems and in normal development.
References
1. Holliday, R., The inheritance of epigenetic defects. Science, 1987. 238(4824): p. 163-170.
2. Waddington, C.H., Genetic assimilation of the bithorax phenotype. Evolution, 1956. 10: p. 1-13.
3. Jablonka, E. and G. Raz, Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol, 2009. 84(2): p. 131-76.
4. Heard, E. and R.A. Martienssen, Transgenerational epigenetic inheritance: myths and mechanisms. Cell, 2014. 157(1): p. 95-109.
5. Johannes, F., et al., Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet, 2009. 5(6): p. e1000530.
6. Becker, C., et al., Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature, 2011. 480(7376): p. 245-9.
7. Schmitz, R.J., et al., Transgenerational epigenetic instability is a source of novel methylation variants. Science, 2011. 334(6054): p. 369-73.
8. Morgan, H.D., et al., Epigenetic inheritance at the agouti locus in the mouse. Nat Genet, 1999. 23(3): p. 314-8.
9. Greer, E.L., et al., Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature, 2011. 479(7373): p. 365-71.
10. Cavalli, G. and R. Paro, The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell, 1998. 93(4): p. 505-18.
11. Seong, K.H., et al., Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell, 2011. 145(7): p. 1049-61.
12. Bantignies, F., et al., Inheritance of Polycomb-dependent chromosomal interactions in Drosophila. Genes Dev, 2003. 17(19): p. 2406-20.
13. Morgan, H.D., et al., Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet., 1999. 23(3): p. 314-318.
14. Rassoulzadegan, M., et al., RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature, 2006. 441(7092): p. 469-74.
15. Onorati, M.C., et al., Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes. PLoS Genet, 2015. 11(8): p. e1005444.
16. Dorn, R., et al., The enhancer of position-effect variegation of Drosophila, E(var)3-93D, codes for a chromatin protein containing a conserved domain common to several transcriptional regulators. Proc Natl Acad Sci U S A, 1993. 90(23): p. 11376-80.
17. Klosin, A., et al., Transgenerational transmission of environmental information in C. elegans. Science, 2017. 356(6335): p. 320-323.
18. Schuettengruber, B., et al., Genome Regulation by Polycomb and Trithorax: 70 years and counting. Cell, 2017. in press, Sept 21 issue.
19. Piunti, A. and A. Shilatifard, Epigenetic balance of gene expression by Polycomb and COMPASS families. Science, 2016. 352(6290): p. aad9780.
20. Schuettengruber, B., et al., Genome Regulation by Polycomb and Trithorax Proteins. Cell, 2007. 128(4): p. 735-745.
21. Entrevan, M., B. Schuettengruber, and G. Cavalli, Regulation of Genome Architecture and Function by Polycomb Proteins. Trends Cell Biol, 2016.
22. Cavalli, G. and R. Paro, Epigenetic inheritance of active chromatin after removal of the main transactivator. Science, 1999. 286(5441): p. 955-8.
23. Greer, E.L., et al., Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature, 2010. 466(7304): p. 383-7.
24. Inoue, A., et al., Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature, 2017.
25. Ciabrelli, F., et al., Stable Polycomb-dependent transgenerational inheritance of chromatin states in Drosophila. Nat Genet, 2017. 49(6): p. 876-886.
26. Bonev, B. and G. Cavalli, Organization and function of the 3D genome. Nat Rev Genet, 2016. 17(11): p. 661-678.
27. Dekker, J. and L. Mirny, The 3D Genome as Moderator of Chromosomal Communication. Cell, 2016. 164(6): p. 1110-21.
28. Dixon, J.R., D.U. Gorkin, and B. Ren, Chromatin Domains: The Unit of Chromosome Organization. Mol Cell, 2016. 62(5): p. 668-80.
29. Sexton, T., et al., Three-dimensional folding and functional organization principles of the Drosophila genome. Cell, 2012. 148(3): p. 458-72.
30. Lanctot, C., et al., Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet, 2007. 8(2): p. 104-15.
31. Lanzuolo, C., et al., Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat Cell Biol, 2007. 9(10): p. 1167-74.
32. Schuettengruber, B., et al., Cooperativity, Specificity, and Evolutionary Stability of Polycomb Targeting in Drosophila. Cell Rep, 2014.
33. Dixon, J.R., et al., Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature, 2012. 485(7398): p. 376-80.
34. Hou, C., et al., Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol Cell, 2012. 48(3): p. 471-84.
35. Nora, E.P., et al., Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature, 2012. 485(7398): p. 381-5.
36. Bantignies, F., et al., Polycomb-Dependent Regulatory Contacts between Distant Hox Loci in Drosophila. Cell, 2011. 144(2): p. 214-26.
37. Bonev, B., et al., Multi-scale 3D genome rewiring during mouse neural development. Cell, 2017. in press.
38. Schoenfelder, S., et al., Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat Genet, 2015.
39. Pirrotta, V., Of giraffes' necks and the inheritance of chromatin states. Nat Genet, 2017. 49(6): p. 821-823.


