Projet : The role of Polycomb proteins in development and tumorigenesis
Coordination between cellular proliferation and differentiation ensures proper tissue morphogenesis and maintains homeostasis in multicellular organisms. Appropriate numbers of undifferentiated cells must be generated at specific developmental stages and these cells must exit the cell cycle in a tightly regulated manner to ensure proper cell fate specification and pattern formation.
The prevailing paradigm posits that Polycomb Group (PcG) proteins maintain stem cell identity by repressing differentiation genes. Mutation or misexpression of PcG genes has been associated with several types of human cancer. Polycomb group (PcG) proteins form two main epigenetic transcription repressor complexes, PRC2 and PRC1, highly conserved from fly to humans, which generally coregulate their target genes. They colocalize almost perfectly during embryogenesis, and their embryonic phenotypes are similar, with posterior homeotic transformations due to misexpression of homeotic Hox genes. Later in development, alterations in PcG components induce cancer (Figure 3), suggesting that PcG proteins may be dynamically recruited to new target genes. PcG proteins additionally bind and regulate genes implicated in major signaling pathways and therefore also participate in dynamic gene regulatory processes.
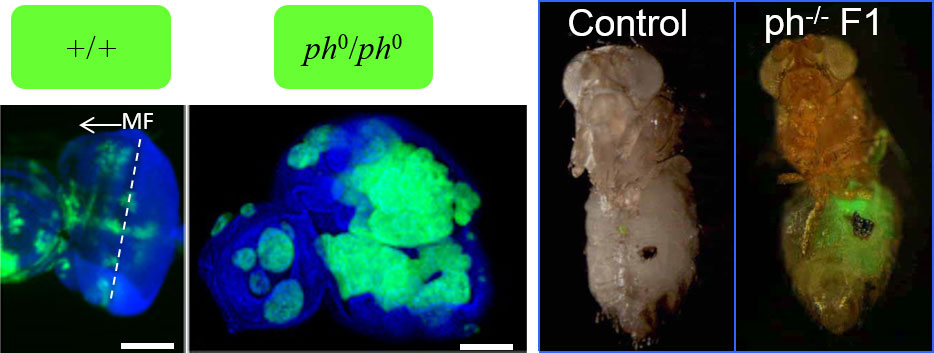
Using as a model the Drosophila larval imaginal eye disc, which shares critical features with mammalian epithelial tissues, we demonstrated that mutations affecting PRC1 subunits, but not PRC2, trigger neoplastic tumours in the larval imaginal discs. PRC1 components act as neoplastic tumor suppressors independently of PRC2 function.
by specifically targeting a thousand of new genes during larval stages of fly development. We named these non-canonical genes, “Neo-PRC1”; they massively outnumber canonical targets, are devoid of the H3K27me3 epigenetic mark and carry instead the active mark H3K27Ac. Remarkably, neo-PRC1 genes are mainly involved in the regulation of cell proliferation, differentiation, signaling and polarity. Alterations in PRC1 components specifically deregulate this set of genes, whereas canonical targets are derepressed in both PRC1 and PRC2 mutants. Together, these results suggest that the mechanism of recruitment of PRC1 on its neo-sites is independent of PRC2 and depends on new molecular mechanisms that remain to be determined. The search for these mechanisms and of their molecular significance in flies and mammals is the basis of our current interest.